September 21, 2024, marked the seventh anniversary of the significant amendments to the Patented Medicines (Notice of Compliance) Regulations (Regulations). This article provides an update on activities in the seventh year following the amendments, including new actions and Court decisions, both on the merits and procedural. Our sixth anniversary update is available with links to prior year updates.
Status of section 6(1) actions under the Regulations
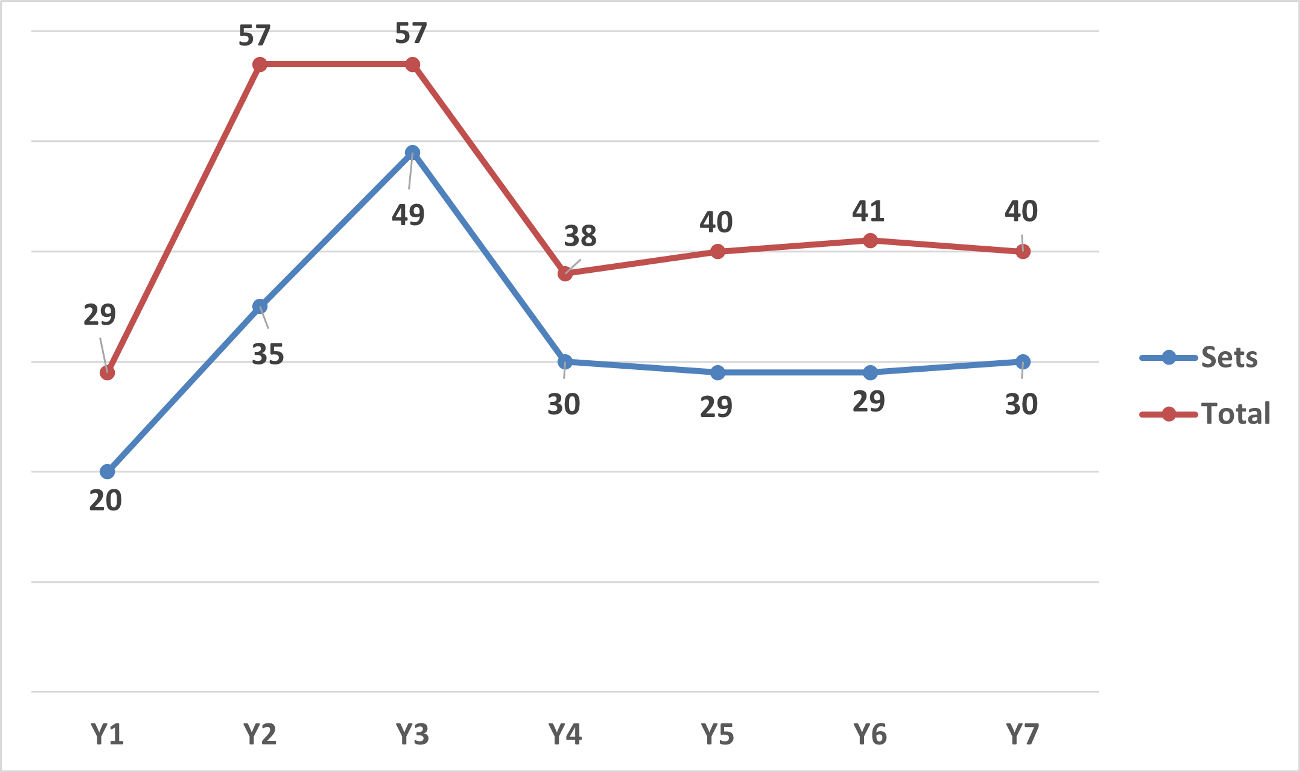
From September 21, 2023 to September 20, 2024, approximately 30 sets1 of actions (40 total actions) under section 6(1) were started, which is consistent with the annual number cases from 2020-2024. A comparison to previous years is provided below:
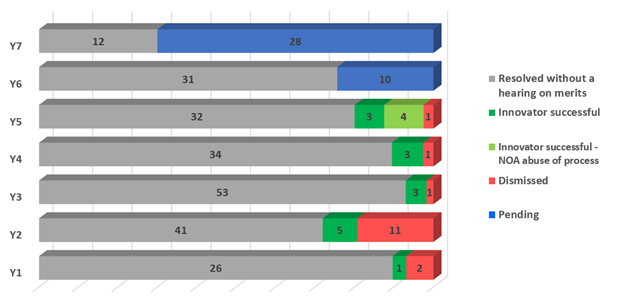
Of the approximately 302 actions started since the amendments, the majority continue to be resolved without completing a hearing on the merits of infringement and/or invalidity (229, in grey below).
To date, 31 actions were pursued to decisions on the merits of infringement and/or invalidity, with the innovator successful in 15 actions. There are therefore 19 decisions on the merits, 12 of which were in favour of the innovator (the Court issued a single decision for some actions heard together and issued two decisions—one each for infringement and invalidity—in one action).
Thus far, no trial decision has been overturned on appeal. There is one appeal pending in the Federal Court of Appeal. On September 19, the Supreme Court of Canada (SCC) granted Pharmascience the first leave to appeal under the amended Regulations. The appeal relates to the patentability of methods of medical treatment.
The following 21 drugs have at least one action ongoing:
- abacavir, dolutegravir, lamivudine (Viiv’s TRIUMEQ)
- aflibercept (Bayer’s EYLEA)
- axitinib (Pfizer’s INLYTA)
- brexpiprazole (Otsuka’s REXULTI)
- canagliflozin (Janssen’s INVOKANA)
- carfilzomib (Amgen’s KYPROLIS)
- cladribine (EMD Serono’s MAVENCLAD)
- diclofenac (Aralez’s CAMBIA)
- dolutegravir (Viiv’s TIVICAY)
- doxepin (Paladin’s SILENOR)
- eculizumab (Alexion’s SOLIRIS)
- empagliflozin (Boehringer’s JARDIANCE)
- esomeprazole / naproxen (Nuvo’s VIMOVO)
- lenvatinib (Eisai’s LENVIMA)
- nilotinib (Novartis’ TASIGNA)
- nintedanib (Boehringer’s OFEV)
- regorafenib (Bayer’s STIVARGA)
- rituximab (Roche’s RITUXAN)
- ruxolitinib (Novartis’ JAKAVI)
- trifluridine / tipiracil (Taiho’s LONSURF)
- vortioxetine (Lundbeck’s TRINTELLIX)
In the last year, based on the number of drugs, JAMP was the most active challenger (defendant in seven new actions relating to six drugs), followed by Apotex (defendant in seven new actions relating to five drugs).
Section 8.2 actions: in the past year, fourteen actions were commenced under section 8.2 (for a total of 37 commenced since the amendments to the Regulations). Section 8.2 permits a first person/patentee to bring an infringement action asserting an unaddressed patent once served with a Notice of Allegation. Thirteen actions are ongoing, relating to aflibercept (Bayer’s EYLEA), brexpiprazole (Otsuka’s REXULTI), diclofenac (Aralez’s CAMBIA), and regorafenib (Bayer’s STIVARGA).
Section 8.1 actions: biosimilar manufacturers have also commenced actions seeking declarations of invalidity and non-infringement of patents either before filing a new drug submission or pursuant to section 8.1 of the Regulations. Section 8.1 deems a person who files a submission for a notice of compliance who has reasonable grounds to believe the drug might be alleged to infringe a patent, an ‘interested person’ who may bring an action for a declaration of patent invalidity.
In the last year, Samsung Bioepis commenced an action seeking a declaration of invalidity of a patent relating to ustekinumab (Janssen’s STELARA). Samsung Bioepis and Amgen have ongoing actions seeking declarations of invalidity of a patent relating to eculizumab (Alexion’s SOLIRIS).
Trial decisions on the merits
While no trial decisions were released in the sixth year, the seventh year brought the release of four decisions on the merits, including as set out in our mid-year highlights:
- AbbVie Corporation v JAMP Pharma Corporation (adalimumab, HUMIRA, SIMLANDI) – the Federal Court (FC) found two adalimumab dosing regime patents for treating inflammatory bowel disease and hidradenitis suppurativa invalid for obviousness. The third patent claiming an aqueous formulation of adalimumab was found valid and JAMP conceded infringement. The FC declined to grant a permanent injunction in view of the public interest as switching to other biosimilars could cause increased injection site pain. The trial related to four actions, including two actions pursuant to section 6(1) of the Regulations; the latter two actions were not at issue as Health Canada determined that JAMP was not a ‘second person’; AbbVie’s previous judicial review on whether JAMP was a ‘second person’ was dismissed. AbbVie’s appeal and JAMP’s cross-appeal of the FC trial decision is pending.
- Allergan Inc v Juno Pharmaceuticals Corp (bimatoprost, LUMIGAN RC) – the FC granted Allergan’s action under the Regulations. The asserted formulation claims were found valid (not obvious, disclosure not insufficient); Juno conceded infringement. Juno discontinued its appeal.
- Takeda Canada Inc v Apotex Inc (dexlansoprazole, DEXILANT) – Takeda’s action was dismissed. The claims relating to a pulsatile release formulation of dexlansoprazole were found not infringed and invalid for lack of sound prediction of utility and insufficiency.
- Boehringer Ingelheim (Canada) Ltd v JAMP Pharma Corporation (nintedanib, OFEV) – the FC granted Boehringer’s action in respect of one of two asserted patents. The successful patent claims relate to the use of nintedanib and its esylate salt in the prevention or treatment of idiopathic pulmonary fibrosis, which the Court found valid (not obvious, soundly predicted and not invalid for double patenting) and directly infringed (JAMP had conceded it would induce infringement).
Federal Court of Appeal (FCA) decisions on the merits
- Apotex Inc v Janssen Inc (macitentan, OPSUMIT) – the FCA dismissed Apotex’s appeal, finding Apotex would induce infringement of the 770 patent claims relating to macitentan in combination with a phosphodiesterase type-5 inhibitor (PDE5-I) to treat pulmonary arterial hypertension including based on references in its product monograph to the SERAPHIN study, the pivotal trial regarding monotherapy and combination therapy with a PDE5-I. Apotex’s SCC leave application was dismissed.
- Sandoz Canada Inc v Janssen Inc (macitentan, OPSUMIT) – the FCA dismissed Sandoz’s appeal, finding that the 770 claims were not invalid based on lack of sound prediction, confirming that the threshold for sound prediction is not high.
- Apotex Inc v Janssen Inc (paliperidone palmitate, INVEGA SUSTENNA) – the FCA dismissed Apotex’s appeal, finding Apotex would induce infringement of the 335 patent claims relating to dosing regimens for long-acting paliperidone palmitate depot formulations for treatment of schizophrenia. Apotex’s non-infringement position was premised on not seeking approval for the 75 mg strength syringe, which corresponded to an essential element of the claims, and specifically, that prescribing practices would not change. Apotex’s SCC leave application was dismissed.
- Pharmascience Inc v Janssen Inc (paliperidone palmitate, INVEGA SUSTENNA) – the FCA dismissed Pharmascience’s appeal, finding Pharmascience would induce infringement of the 335 patent claims relating to a dosing regime. Pharmascience’s non-infringement position was premised on not seeking approval for the 75 mg strength syringe, and specifically, that any activities using Pharmascience’s product that would fall within the scope of the claims would be licensed by Janssen, as it provides the 75 mg-eq dose. Pharmascience’s SCC leave application was dismissed.
- Pharmascience Inc v Janssen Inc (paliperidone palmitate, INVEGA SUSTENNA) – the FCA dismissed Pharmascience’s appeal of a decision in a s. 6(1) action under the Regulations finding that claims to a dosing regime were not invalid as a method of medical treatment (non-obviousness finding was not pursued on appeal). Pharmascience’s SCC leave application was granted. Assuming average timelines, it is anticipated the Supreme Court will hear the appeal in May or September 2025, and render a decision by early 2026.
Other Court decisions
In addition, a number of decisions were released in the past year which provide interpretation on aspects of the Regulations:
- Janssen Inc v Apotex Inc (paliperidone palmitate, INVEGA SUSTENNA) – reversing the decision of the FC, the FCA held that it was an abuse of process for Apotex to raise invalidity in defending actions under the Regulations after unsuccessfully asserting only non-infringement in a 2021 action involving the same parties and the same patent. The FCA observed that a principal aim of the amended Regulations was to avoid multiple proceedings and, had Apotex wished to allege invalidity, it could and should have done so in the 2021 action.
- Bayer Inc v BGP Pharma ULC (Viatris Canada) (aflibercept, EYLEA, YESAFILI) – the FCA affirmed an FC decision, which found that the Minister of Health’s determination that Biosimilar Collaborations Ireland Limited was entitled to the benefit of section 5 of Regulations, including prior service of a Notice of Allegation by the previous owner of the new drug submission for YESAFILI (an aflibercept biosimilar) was reasonable.
- Janssen Inc v Canada (Health) (ustekinumab, STELARA) – the FCA dismissed Janssen’s appeal regarding the eligibility of a patent for listing on the Patent Register against two supplementary new drug submissions.
Section 8 actions
To date, there have been no amended section 8 decisions on the merits. Three section 8 actions remain pending:
- abiraterone (commenced by Pharmascience) – trial scheduled for May 2025.
- fampridine (commenced by Pharmascience) – trial not scheduled, expected in the first quarter of 2026.
- fampridine (commenced by Taro Pharmaceuticals) – trial not scheduled.
Reference
1. Actions between the same parties regarding the same innovator reference drug are considered one “set”. The counts are provided as of the seven-year anniversary, September 21, 2024.
Should you have any questions, please do not hesitate to contact a member of the Pharmaceutical Litigation group.
The preceding is intended as a timely update on Canadian intellectual property and technology law. The content is informational only and does not constitute legal or professional advice. To obtain such advice, please communicate with our offices directly.
Related Publications & Articles
-
Federal Court rules on patent listing again, confirms generic not required to address patent submitted before ANDS filing but listed after
On January 20, 2025, Justice O’Reilly of the Federal Court dismissed Bayer’s judicial review of the Minister of Health’s decision to list Canadian Patent No. 2,970,315 (315 Patent) on the Patent Regis...Read More -
Minister of Health announces new bilateral agreements with provinces for rare disease drugs
Canada’s first-ever National Strategy for Drugs for Rare Diseases includes up to $1.4 billion in funding for provinces and territories, to be negotiated through bilateral agreements.Read More -
SCC to revisit “method of medical treatment” patent claims
This spring, the Supreme Court of Canada (SCC) will consider the scope of patentable subject-matter as it relates to “methods of medical treatment”.Read More
