As we previously reported, on December 19, 2024, the Patented Medicine Prices Review Board (PMPRB) released its Draft Guidelines for PMPRB Staff regarding Administrative Process for Excessive Price Hearing Recommendation (Draft Guidelines), together with a Simplified Overview.
The general review processes contemplated by the Draft Guidelines have been discussed previously in our summary of the proposed framework. As illustrated in Figure 1 of the Draft Guidelines (reproduced below), the review process consists of two screening steps:
- an Initial Review or Annual Review that applies to all patented medicines (with limited exceptions), and
- an In-Depth Review, which can be the result of a referral following the preceding step or a complaint from an approved individual or organization.
PMPRB Staff may recommend a hearing to the Chairperson following an In-Depth Review. The Chairperson then decides whether to close the In-Depth Review (with or without an Undertaking) or to issue a Notice of Hearing to determine whether a patented medicine is being or has been sold at an excessive price.
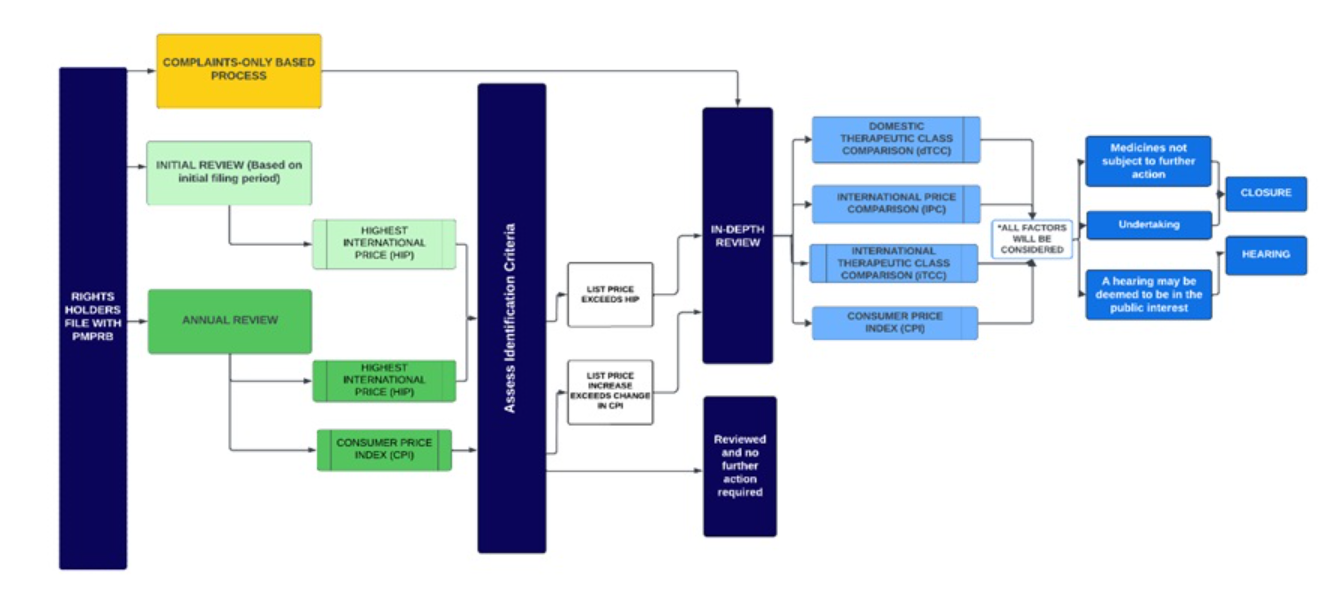
The following points from the Draft Guidelines are notable; some of these were the subject of Phase 2 Consultations following the release of the Discussion Guide:
- International Price Comparison against Highest International Price (HIP): both the Initial Review and subsequent Annual Reviews of a patented medicine compare the Canadian list price against the HIP among the basket of comparator countries (“PMPRB11”). The Draft Guidelines note, however, that the selection of HIP is based on “administrative efficiency and resource prioritization” and does not presuppose that prices above or below the HIP are excessive or not excessive. Such a determination can only be made by Hearing Panels considering factors in subsection 85(1) of the Patent Act.
- One-year actual Consumer Price Index (CPI): during an Annual Review, list prices are also reviewed against the change in the CPI each year using the one-year lagged CPI. An increase of the list price by an amount greater than the change in CPI would result in an In-Depth Review unless there was no price increase in the previous year and the increase in the second year is lower than or equal to the total change in CPI over those two years.
- Deferral letters: in the event of resource constraints, some patented medicines with list prices above the HIP may receive deferral letters while the PMPRB Staff prioritize those with list prices significantly above the HIP or whose list price increases are significantly above the CPI. They are, however, not relieved from the calculation of excess revenue that may accrue during the period of deferral.
- Transition period: new medicines (i.e., patented medicines first sold on or after July 1, 2022) and existing medicines (i.e., those first sold before July 1, 2022) are subject to the same Annual Review process. Existing medicines will be reviewed starting one year from the date the final Guidelines go into effect.
- In-Depth Review: patented generic medicines, over-the-counter medicines and veterinary medicines are only subject to an In-Depth Review if a complaint is received. For all other patented medicines, the receipt of a complaint from an approved individual or organization (see below) automatically leads to an In-Depth Review. Separately, if an In-Depth Review results from an Initial or Annual Review, rights holders are advised within 60 days of the filing deadline for their semi-annual filing.
An In-Depth Review consists of both a scientific review (i.e., Therapeutic Class Comparison, or TCC) and a price review, which take place in parallel. With respect to the TCC, rights holders are encouraged to submit their input as soon as possible after being notified of an In-Depth Review. Rights holders may also provide input to the pricing team within 3 months of being notified of an In-Depth Review. The In-Depth Review process could take between 12 and 28 months, depending on its complexity.
- Complaints: the approved individuals or organizations whose complaints lead to In-Depth Reviews are:
- the Federal Minister of Health or any of their Provincial or Territorial counterparts;
- publicly-funded drug programs; or
- Canadian life and health insurance companies and their trade association(s).
- Undertakings and settlement proposals: the PMPRB reports publicly on all Undertakings accepted by the Chairperson and all settlements accepted by Hearing Panels. The information reported ordinarily includes the name of the patented medicine and/or the rights holder and such other information as is considered appropriate.
The deadline for providing submissions on the Draft Guidelines is March 19, 2025. The PMPRB continues to plan to release its final Guidelines in 2025.
Should you have any questions, please do not hesitate to contact a member of the Life Sciences Regulatory & Compliance Group.
The preceding is intended as a timely update on Canadian intellectual property and life sciences regulatory law. The content is informational only and does not constitute legal or professional advice. To obtain such advice, please communicate with our offices directly.
Related Publications & Articles
-
Minister of Health announces new bilateral agreements with provinces for rare disease drugs
Canada’s first-ever National Strategy for Drugs for Rare Diseases includes up to $1.4 billion in funding for provinces and territories, to be negotiated through bilateral agreements.Read More -
Government of Canada announces first pharmacare agreements
The Minister of Health recently announced the first of these bilateral agreements with Manitoba ($219 million over four years), British Columbia ($670 million over four years) and Prince Edward Island...Read More -
PMPRB announces Anie Perrault as acting Chairperson and releases latest expenditure report on private drug plan costs and utilization
On March 6, 2025, the Patented Medicine Prices Review Board announced that Thomas Digby stepped down from his role as Chairperson of the PMPRB. Vice-Chairperson Anie Perrault is now serving as acting ...Read More
