UPDATE: See written submissions by stakeholders regarding the Discussion Guide and the PMPRB’s August 13, 2024 webinar slides. See also new Draft Guidelines.
As previously reported, the Patented Medicine Prices Review Board (PMPRB) published a Discussion Guide for Phase 2 Consultations on New Guidelines as part of a three-phase consultation process that started in September 2023. This article provides an overview of the price review framework proposed by the PMPRB and key topics on which stakeholder feedback is sought.
Overview of the proposed framework
The following schematic1 illustrates the basic structure of the proposed price review process, which consists of four main parts (“Sections”, each of which is circled).
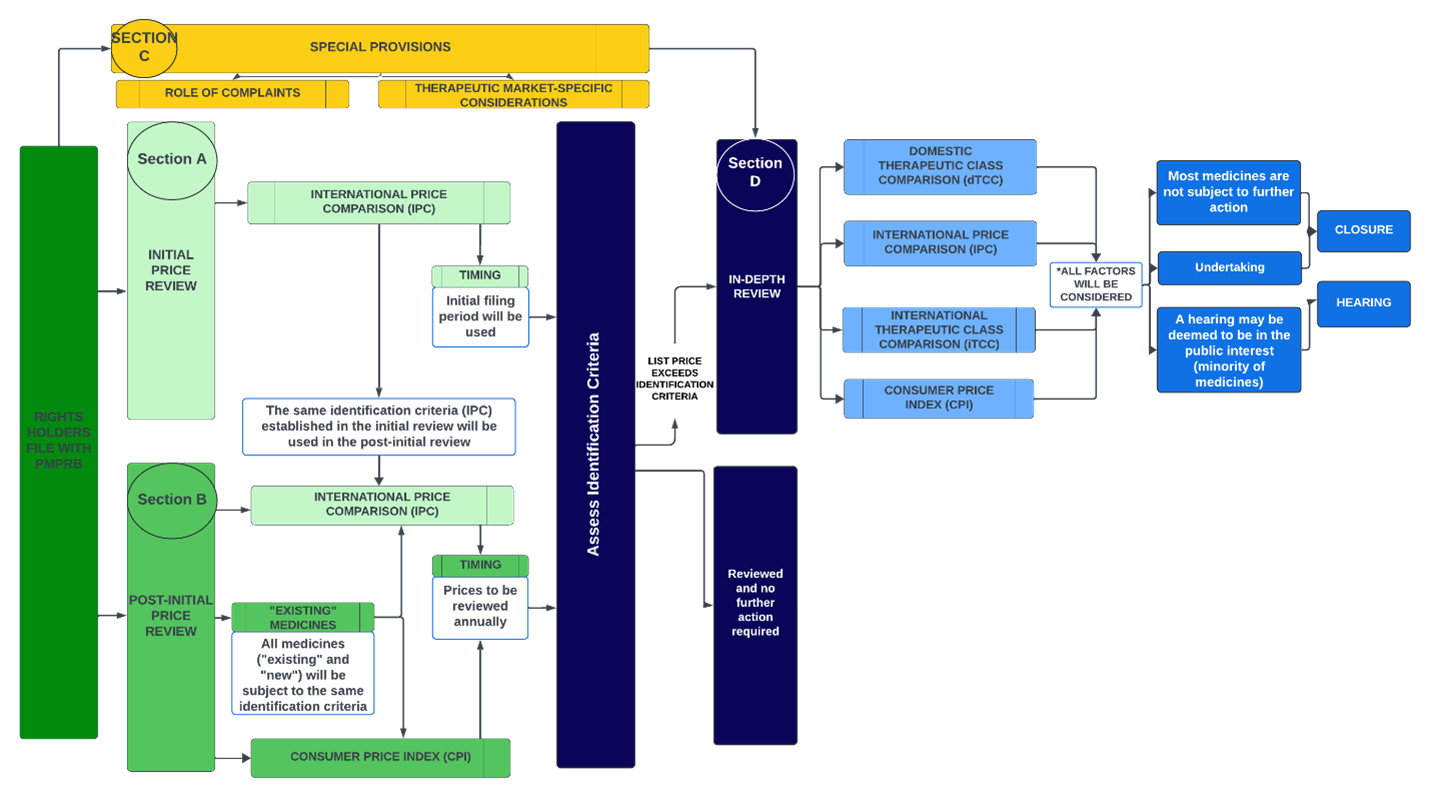
As described in more detail below, under the proposed approach, all medicines would be subject to initial review (using international prices only; Section A). Only medicines which progress to the in-depth review stage (Section D) either following initial review or annual post-initial price review (Section B) would be subject to a full review using international prices, Consumer Price Index (CPI), and therapeutic class price comparisons. The Board is also considering using complaints (Section C) as an additional pathway leading to an in-depth review.
Section A: initial price review
The Patented Medicines Regulations require that Rights Holders report price and sales data to the PMPRB within 30 days after the medicine is first sold in Canada, and on a semi-annual basis moving forward. Under the proposed framework, the PMPRB will use a medicine’s first semi-annual filing to conduct an initial price review based on the International Price Comparison (IPC) identification criteria and flag medicines at a greater risk of excessive pricing for a more in-depth review. Those at low risk would receive the initial review within 60 days and require no further consideration until the post-initial price review on an annual basis, as discussed below (Section B).
The PMPRB has identified the following three potential IPC identification criteria and seeks feedback on the price level within the basket of comparator countries (PMPRB11) that should be used for the initial review (which would then be used for all subsequent post-initial reviews) (Discussion Guide Section 6.1.1, “Topic 1”):
- Median International Price (MIP)
- Highest International Price (HIP), or
- The midpoint between the MIP and HIP.
For context, the Discussion Guide also provides some data, including that 78% of all patented medicines had Canadian list prices higher than the MIP in 2023 and 32% were higher than the HIP.
In contrast to the current Guidelines, the proposal reserves the use of domestic Therapeutic Class Comparison (dTCC) and international Therapeutic Class Comparison (iTCC) for in-depth review only.
Section B: post-initial price review
On an annual basis, the PMPRB will continue to monitor medicines that have already undergone initial price review by comparing list prices against (a) IPC identification criteria, and (b) changes in the CPI. If identification criteria are not met, no further action will be conducted by PMPRB Staff until the following annual review or if a complaint is received. If the list price exceeds identification criteria, Staff would proceed to an in-depth review.
CPI increase criteria: The PMPRB is considering two options to “better reflect how price increases have historically evolved in Canada”— comparison against one-year actual CPI, or against combined CPI for two years (if the list price is increased in the second year) (Discussion Guide Section 6.1.3, “Topic 3”).
No distinction between “new” and “existing” medicines, transitional period: As part of the proposed review process, the PMPRB would not distinguish between medicines with a maximum average potential price or projected non-excessive average price as of July 1, 2022 (Existing Medicines) and those without (New Medicines). However, Existing Medicines with list prices above the IPC identification criteria will be given a transitional period – a period of time to adapt to the new Guidelines before an in-depth review will be commenced. In this regard, the PMPRB is seeking feedback on the length of any transitional period, with options from one year to three years. (Discussion Guide Section 6.1.2, “Topic 2”).
Section C: special provisions
In addition to the above mechanism, the PMPRB is considering using the receipt of a pricing complaint as a separate process to identify medicines that warrant in-depth review. Under the proposed framework, for medicines with reduced reporting obligations (i.e., patented over-the-counter medicines, certain non-prescription controlled substances, generic and veterinary medicines), an in-depth review will only be commenced if a complaint is received.
To balance inclusivity and transparency with the need to prevent misuse and to ensure that instances of potential excessiveness can be identified, the PMPRB is considering options for defining who can submit a complaint that automatically leads to an in-depth review (Discussion Guide Section 6.2.1, “Topic 4”).
In addition, the PMPRB is considering expanding the list of medicines eligible for the complaint-only based process to prioritize higher-risk cases (Discussion Guide Section 6.2.2, “Topic 5”). The two options under consideration are:
- The PMPRB will treat patented biosimilars and/or vaccines the same as other medicines, or
- The PMPRB will only open an in-depth review for biosimilars and/or vaccines when a complaint is received.
Section D: in-depth review
An in-depth review would consider all the factors outlined in s. 85(1) of the Patent Act, including comparing list prices to the IPC, changes in CPI, dTCC, and iTCC. For a given TCC, a list of potential therapeutic comparators would be created by the scientific Staff at PMPRB independently from any pricing information. The list would then be returned to the pricing group with relevant scientific context to help understand key points of comparison.
The PMPRB is seeking feedback on two aspects of the scientific review process. First, the PMPRB is considering options to contextualize the degree of similarity of comparators (Discussion Guide Section 6.3.1, “Topic 6”). Second, the PMPRB is deciding whether the Human Drug Advisory Panel (HDAP), an advisory body comprised of independent health professionals, should be optionally engaged at the request of the scientific Staff, or whether HDAP could be eliminated altogether (Discussion Guide Section 6.3.2, “Topic 7”).
After individually assessing the s. 85(1) factors, the pricing review Staff would then balance all the factors together. The in-depth review process could lead to a recommendation to the Chairperson that said review be closed, or alternatively, a Notice of Hearing. In the latter scenario, the Rights Holder would receive a notice of the recommendation and have 3 months to respond before the Chairperson decides whether to issue the Notice of Hearing. In the interim, the Rights Holder may choose to submit a voluntary undertaking to adjust its price and/or pay an amount of money to the Receiver General. Once a Notice of Hearing is issued, however, any proposed voluntary undertaking can no longer be considered by the Staff or Chairperson, and only the hearing panel can consider any proposed settlement terms.
Potential excess revenues will not be calculated as part of the in-depth review. However, any such excess revenues included in proposed voluntary undertakings will continue to be based on the average price per package and/or net revenue information, not the list prices.
Process timelines
The Discussion Guide also includes proposed process timelines and notification steps. For example, Staff would send a letter to Rights Holders within 60 days of receipt of their initial or semi-annual filings indicating whether any of their medicines will be subject to an in-depth review. The in-depth review could be completed “relatively quickly (within approximately 12 months)” or “could take longer (up to approximately 28 months)” depending on complexity.
Next steps
The PMPRB is requesting written stakeholder feedback on the topics set out above by September 11, 2024. A webinar will be hosted by the PMPRB on August 13, 2024 to answer questions about the Discussion Guide.
According to the Discussion Guide, the PMPRB intends to publish new draft Guidelines by the end of 2024 after considering the feedback received on the two previous phases. These draft Guidelines will be open for a Notice and Comment period before finalization and implementation, anticipated in 2025.
Should you have any questions, please do not hesitate to contact a member of the Life Sciences Regulatory & Compliance Group.
The preceding is intended as a timely update on Canadian intellectual property and life sciences regulatory law. The content is informational only and does not constitute legal or professional advice. To obtain such advice, please communicate with our offices directly.
Reference
Related Publications & Articles
-
Minister of Health announces new bilateral agreements with provinces for rare disease drugs
Canada’s first-ever National Strategy for Drugs for Rare Diseases includes up to $1.4 billion in funding for provinces and territories, to be negotiated through bilateral agreements.Read More -
Government of Canada announces first pharmacare agreements
The Minister of Health recently announced the first of these bilateral agreements with Manitoba ($219 million over four years), British Columbia ($670 million over four years) and Prince Edward Island...Read More -
PMPRB announces Anie Perrault as acting Chairperson and releases latest expenditure report on private drug plan costs and utilization
On March 6, 2025, the Patented Medicine Prices Review Board announced that Thomas Digby stepped down from his role as Chairperson of the PMPRB. Vice-Chairperson Anie Perrault is now serving as acting ...Read More
